IS LEUKEMIA A TYPE OF CANCER?
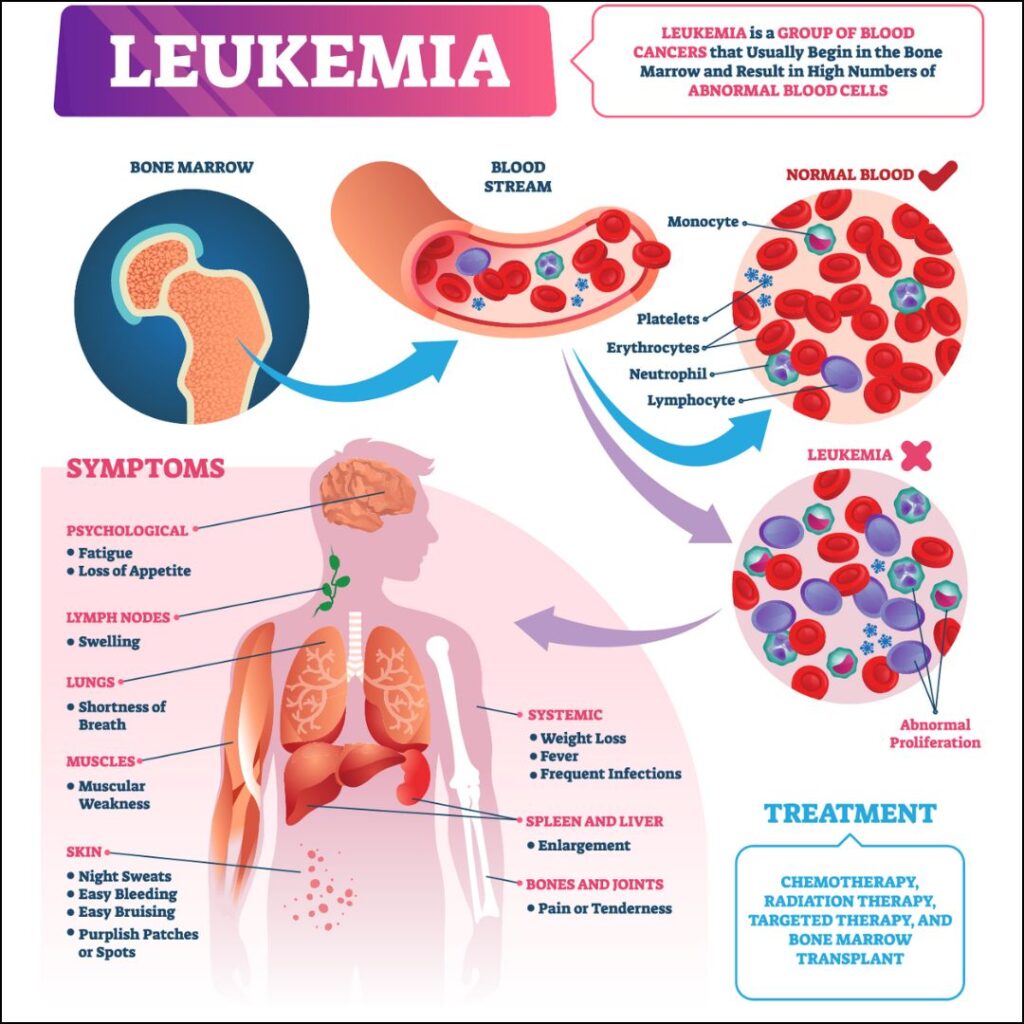
Leukemia is a group of blood cancers that typically begin in the bone marrow and result in the production of abnormal white blood cells. These abnormal cells outnumber the healthy cells, leading to a range of health issues, including anemia, bleeding, and infections. Leukemia is categorized into different types based on the speed of progression (acute or chronic) and the type of white blood cells affected (lymphoid or myeloid). This comprehensive research paper delves into the classification, pathophysiology, clinical presentation, diagnosis, treatment, and current research in leukemia.
CLASSIFICATION OF LEUKEMIA.
Leukemia is broadly classified into four main types, each with distinct characteristics:
- Acute Lymphoblastic Leukemia (ALL):
- Overview: ALL is the most common type of leukemia in children, but it also affects adults. It is characterized by the rapid production of immature lymphoblasts (a type of white blood cell).
- Subtypes: ALL is further classified based on the immunophenotype of the blast cells, including B-cell ALL and T-cell ALL.
- Prognosis: The prognosis for ALL has improved significantly, especially in pediatric cases, with long-term survival rates exceeding 85% in children.
- Acute Myeloid Leukemia (AML):
- Overview: AML is characterized by the rapid accumulation of myeloblasts in the bone marrow, which interfere with the production of normal blood cells. AML is more common in adults, particularly the elderly.
- Subtypes: AML is classified into several subtypes based on genetic abnormalities and the morphology of the blast cells, as per the World Health Organization (WHO) classification.
- Prognosis: The prognosis for AML varies depending on the patient’s age, genetic mutations, and response to treatment. The overall 5-year survival rate is about 25-30%.
- Chronic Lymphocytic Leukemia (CLL):
- Overview: CLL is a slow-progressing leukemia that primarily affects older adults. It involves the accumulation of mature but functionally incompetent lymphocytes, usually B-cells.
- Prognosis: CLL has a highly variable prognosis, with some patients living for decades without requiring treatment, while others may progress more rapidly.
- Chronic Myeloid Leukemia (CML):
- Overview: CML is a slowly progressing leukemia that arises from a clonal expansion of myeloid cells. It is characterized by the presence of the Philadelphia chromosome (a translocation between chromosomes 9 and 22).
- Phases: CML progresses through three phases: the chronic phase, the accelerated phase, and the blast crisis phase. Most patients are diagnosed in the chronic phase.
- Prognosis: The prognosis for CML has dramatically improved with the advent of tyrosine kinase inhibitors (TKIs), with many patients achieving long-term remission.
PATHOPHYSIOLOGY.

The pathophysiology of leukemia involves genetic mutations and chromosomal abnormalities that disrupt normal hematopoiesis, leading to uncontrolled proliferation of abnormal blood cells.
- Genetic Mutations:
- Leukemia is driven by various genetic mutations that affect key regulatory genes involved in cell growth, differentiation, and apoptosis. These mutations can occur de novo or be inherited as part of a genetic predisposition.
- For example, in ALL, common genetic abnormalities include the t(9;22) translocation (Philadelphia chromosome), t(12;21) translocation, and mutations in the IKZF1 and NOTCH1 genes.
- AML is characterized by diverse genetic alterations, including mutations in FLT3, NPM1, and CEBPA, as well as chromosomal translocations such as t(8;21) and inv(16).
- Chromosomal Abnormalities:
- The Philadelphia chromosome, resulting from a translocation between chromosomes 9 and 22, is a hallmark of CML and is also found in a subset of ALL cases. This translocation creates the BCR-ABL fusion gene, which encodes a constitutively active tyrosine kinase that drives leukemogenesis.
- Other chromosomal abnormalities, such as trisomy 12 and deletions of 11q or 17p, are common in CLL and contribute to disease progression.
- Disruption of Hematopoiesis:
- In leukemia, the bone marrow is infiltrated by malignant cells, which impair the production of normal blood cells. This leads to anemia, thrombocytopenia, and neutropenia, resulting in fatigue, bleeding tendencies, and increased susceptibility to infections.
- The accumulation of abnormal leukemic cells in the blood, bone marrow, and other organs such as the liver, spleen, and lymph nodes, further contributes to disease symptoms and complications.
CLINICAL PRESENTATION.
The clinical presentation of leukemia varies depending on the type and stage of the disease but generally includes symptoms related to bone marrow failure and organ infiltration.
- Acute Leukemias (ALL and AML):
- Symptoms: Patients with acute leukemia often present with symptoms related to bone marrow failure, including fatigue, pallor, fever, recurrent infections, easy bruising, and bleeding (such as nosebleeds or gum bleeding). Bone pain and joint pain are also common, especially in children with ALL.
- Organ Involvement: Hepatosplenomegaly (enlarged liver and spleen) and lymphadenopathy (enlarged lymph nodes) are frequently observed. In ALL, central nervous system (CNS) involvement can occur, leading to headaches, seizures, or cranial nerve palsies.
- Chronic Leukemias (CLL and CML):
- Symptoms: Chronic leukemias often have an indolent course and may be asymptomatic for years. When symptoms do occur, they are often nonspecific, such as fatigue, weight loss, night sweats, and an enlarged spleen (splenomegaly).
- Progression: In CLL, disease progression may lead to recurrent infections, autoimmune hemolytic anemia, and transformation to a more aggressive form of lymphoma (Richter’s syndrome). CML progresses through a chronic phase to an accelerated phase, and eventually to a blast crisis phase, which resembles acute leukemia.
DIAGNOSIS.
The diagnosis of leukemia involves a combination of clinical evaluation, laboratory tests, and imaging studies.
- Blood Tests:
- Complete Blood Count (CBC): A CBC is usually the first test performed and may reveal leukocytosis (increased white blood cell count), anemia, and thrombocytopenia. The presence of blasts (immature white blood cells) in the peripheral blood is a key finding in acute leukemia.
- Blood Smear: Examination of a peripheral blood smear under the microscope can provide clues to the diagnosis, such as the presence of blast cells in acute leukemia or the characteristic “smudge cells” in CLL.
- Bone Marrow Biopsy:
- A bone marrow biopsy is essential for the definitive diagnosis of leukemia. It involves aspirating and examining bone marrow tissue to assess cellularity, morphology, and the proportion of blasts. Flow cytometry and cytogenetic analysis can further classify the leukemia subtype and identify specific genetic abnormalities.
- Immunophenotyping:
- Flow cytometry is used to characterize the surface markers on the leukemic cells, which helps differentiate between different types of leukemia. For example, B-cell ALL is positive for CD19, CD20, and CD22, while T-cell ALL expresses CD3, CD7, and CD5.
- Cytogenetic and Molecular Testing:
- Cytogenetic analysis, including karyotyping and fluorescence in situ hybridization (FISH), can identify chromosomal abnormalities such as the Philadelphia chromosome in CML or the t(12;21) translocation in ALL.
- Molecular testing, such as polymerase chain reaction (PCR), can detect specific genetic mutations or fusion genes, such as BCR-ABL in CML or FLT3-ITD in AML.
- Imaging:
- Imaging studies, such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI), may be used to evaluate organ involvement, such as splenomegaly, lymphadenopathy, or CNS infiltration.
TREATMENT.
The treatment of leukemia depends on the type, stage, patient’s age, and overall health, and involves a combination of chemotherapy, targeted therapy, radiation therapy, and hematopoietic stem cell transplantation.
- Chemotherapy:

- Chemotherapy is the cornerstone of treatment for most leukemias, particularly acute leukemias. It involves the use of cytotoxic drugs to kill rapidly dividing cancer cells. Treatment is typically divided into induction, consolidation, and maintenance phases.
- In ALL, standard chemotherapy regimens include drugs such as vincristine, prednisone, asparaginase, and doxorubicin. High-dose methotrexate and cytarabine are used in the consolidation phase, and maintenance therapy may continue for several years.
- AML treatment typically involves induction chemotherapy with cytarabine and an anthracycline (such as daunorubicin or idarubicin), followed by consolidation therapy with additional cycles of chemotherapy or hematopoietic stem cell transplantation.
- Targeted Therapy:
- Targeted therapies have revolutionized the treatment of certain leukemias by specifically targeting genetic mutations or abnormal proteins involved in the disease process.
- Tyrosine Kinase Inhibitors (TKIs): TKIs, such as imatinib, dasatinib, and nilotinib, target the BCR-ABL fusion protein in CML and Philadelphia chromosome-positive ALL, leading to significant improvements in survival and disease control.
- Monoclonal Antibodies: Rituximab, an anti-CD20 monoclonal antibody, is used in combination with chemotherapy for the treatment of CLL and certain types of B-cell ALL.
- FLT3 Inhibitors: FLT3 inhibitors, such as midostaurin and gilteritinib, are used in AML patients with FLT3 mutations to improve outcomes.
- Radiation Therapy:
- Radiation therapy is used in specific situations, such as for the treatment of CNS involvement in ALL or for the palliation of symptoms in advanced CLL or CML.
- Hematopoietic Stem Cell Transplantation (HSCT):
- HSCT, also known as bone marrow transplantation, is a potentially curative treatment for certain types of leukemia, particularly in younger patients with high-risk or relapsed disease.
- Allogeneic HSCT: Involves the transplantation of stem cells from a healthy donor, usually a sibling or matched unrelated donor. It is commonly used in AML, ALL, and CML.
- Autologous HSCT: Involves the transplantation of the patient’s own stem cells after high-dose chemotherapy, primarily used in selected cases of AML and CLL.
- Immunotherapy:
- Immunotherapy, which harnesses the patient’s immune system to fight cancer, is an emerging treatment modality in leukemia.
- CAR T-Cell Therapy: Chimeric antigen receptor (CAR) T-cell therapy involves genetically modifying the patient’s T-cells to target specific antigens on the surface of leukemic cells. CAR T-cell therapy has shown remarkable success in relapsed or refractory ALL and is being explored in other leukemias.
- Immune Checkpoint Inhibitors: These drugs, such as pembrolizumab and nivolumab, target immune checkpoint proteins like PD-1/PD-L1, enhancing the immune response against leukemic cells. They are currently under investigation in clinical trials for leukemia.
Current Research and Future Directions
Research in leukemia is focused on understanding the molecular mechanisms underlying the disease, developing novel targeted therapies, and improving patient outcomes.
- Genomic and Epigenomic Profiling:
- Advances in next-generation sequencing (NGS) are enabling comprehensive genomic and epigenomic profiling of leukemia, uncovering new genetic mutations and pathways involved in leukemogenesis. This knowledge is leading to the development of personalized medicine approaches, where treatment is tailored based on the patient’s specific genetic and molecular profile.
- Minimal Residual Disease (MRD) Monitoring:
- MRD refers to the small number of leukemic cells that remain in the patient after treatment and can lead to relapse. MRD monitoring using sensitive techniques such as flow cytometry, PCR, or NGS is becoming an essential tool for guiding treatment decisions and assessing treatment response in leukemia patients.
- Novel Therapeutic Agents:
- Researchers are actively exploring new therapeutic agents, including small molecule inhibitors, monoclonal antibodies, and immune-based therapies, to target specific molecular abnormalities in leukemia.
- BCL-2 Inhibitors: Venetoclax, a BCL-2 inhibitor, has shown promise in the treatment of CLL and AML by inducing apoptosis in leukemic cells. It is currently being evaluated in combination with other therapies to improve outcomes.
- Bispecific T-cell Engagers (BiTEs): BiTEs are a type of immunotherapy that links T-cells to cancer cells, promoting an immune attack on the leukemia. Blinatumomab, a BiTE targeting CD19 on B-cells, has been approved for the treatment of relapsed or refractory B-cell ALL.
- Microenvironment Targeting:
- The bone marrow microenvironment plays a critical role in leukemia progression and resistance to therapy. Research is ongoing to develop therapies that disrupt the interactions between leukemic cells and their microenvironment, thereby enhancing the effectiveness of existing treatments.
- Clinical Trials:
- Numerous clinical trials are underway to evaluate the safety and efficacy of new therapies and combination regimens in leukemia. Participation in clinical trials offers patients access to cutting-edge treatments and contributes to the advancement of leukemia care.
Conclusion
Leukemia is a complex and heterogeneous group of blood cancers that presents significant challenges in diagnosis and treatment. However, advancements in molecular biology, targeted therapies, and immunotherapy have led to significant improvements in patient outcomes, particularly in certain leukemia subtypes. Ongoing research and clinical trials continue to push the boundaries of knowledge, offering hope for even more effective and personalized treatments in the future. Despite these advancements, leukemia remains a formidable disease, and continued efforts are essential to further understand its biology and develop novel therapeutic strategies that can lead to improved survival and quality of life for patients.
References
- Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551-65.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136-52.
- Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524-37.
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342-50.
- Cortes JE, Pasquini MC, Goldberg SL, et al. Real-world assessment of the effectiveness of dasatinib, imatinib, and nilotinib as first-line treatment of chronic-phase chronic myeloid leukemia: SIMPLICITY. Am J Hematol. 2021;96(1):89-99.
- Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult acute lymphoblastic leukemia depends on response to salvage chemotherapy, allogeneic transplantation in second remission, time to relapse, and age. Cancer. 2012;118(16):3962-71.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(6):721-49.
- Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187-97.
- Jabbour E, Kantarjian H, O’Brien S, et al. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93(3):442-59.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-48.
Written by Fawzi Rufai, Medically Reviewed by Sesan Kareem